Abstract
Constitutive proteasomes (c-20S) are ubiquitously expressed cellular proteases that degrade polyubiquitinated proteins and regulate cell functions. An isoform of proteasome, the immunoproteasome (i-20S), is highly expressed in human T cells, dendritic cells (DCs), and B cells, suggesting that it could be a potential target for inflammatory diseases, including those involving autoimmunity and alloimmunity. Here, we describe DPLG3, a rationally designed, noncovalent inhibitor of the immunoproteasome chymotryptic subunit β5i that has thousands-fold selectivity over constitutive β5c. DPLG3 suppressed cytokine release from blood mononuclear cells and the activation of DCs and T cells, diminished accumulation of effector T cells, promoted expression of exhaustion and coinhibitory markers on T cells, and synergized with CTLA4-Ig to promote long-term acceptance of cardiac allografts across a major histocompatibility barrier. These findings demonstrate the potential value of using brief posttransplant immunoproteasome inhibition to entrain a long-term response favorable to allograft survival as part of an immunomodulatory regimen that is neither broadly immunosuppressive nor toxic.
Sula Karreci E, Fan H, Uehara M, Mihali AB, Singh PK, Kurdi AT, Solhjou Z, Riella LV, Ghobrial I, Laragione T, Routray S, Assaker JP, Wang R, Sukenick G, Shi L, Barrat FJ, Nathan CF, Lin G, Azzi J.
Proc Natl Acad Sci U S A. 2016 Dec 27;113(52):E8425-E8432. doi: 10.1073/pnas.1618548114.
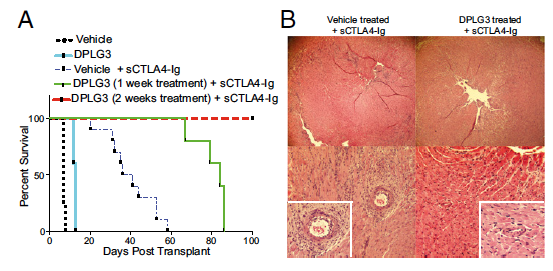
Contribution of β5i inhibition to up-regulation of exhaustion markers on effector T cells and prolonged survival of heart allografts when combined with a single low dose of CTLA4-Ig. (A) C57BL/6 recipients of BALB/c hearts treated with DPLG3 (25 mg/kg daily for 10 d) exhibited prolonged heart allograft survival compared with mice treated with vehicle (MST, 13 vs. 7 d, respectively; n = 4–5 mice/group; *P < 0.01). C57BL/6 recipients of BALB/c hearts treated with DPLG3 (25 mg/kg daily for 7 d) with a single low dose of CTLA4-Ig (sCTLA4-Ig) (250 μg on day 2) exhibited prolonged heart allograft survival compared with mice treated with vehicle and sCTLA4-Ig (MST, 84 and 38.5 d, respectively; n = 5–10 mice/group; P < 0.05). However, C57BL/6 recipients of BALB/c hearts treated both with DPLG3 (25 mg/kg daily for 14 d) and with sCTLA4-Ig had significantly longer allograft survival than mice treated with vehicle and sCTLA4-Ig (MST, >100 and 38.5 d, respectively; n = 5–10 mice/group; P < 0.05). (B) Representative examples of cardiac allograft histology at day 28 post transplant show significantly lower grades of acute cellular rejection (H&E stain; original magnification, 10Å~) in mice treated with DPLG3 and sCTLA4-Ig compared with mice treated with vehicle and sCTLA4-Ig (n = 6 mice per group).