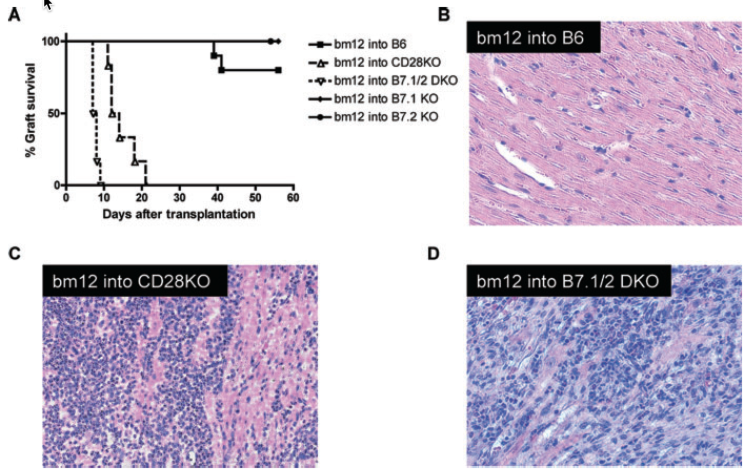
Blockade of the B7: CD28 costimulatory pathway has emerged as a promising therapy to prevent allograft rejection. However, this pathway has also been demonstrated to be important for the generation and maintenance of regulatory T cells. In this study, we investigated the role of the B7: CD28 pathway in the ‘bm12 into B6’ MHC class II-mismatched vascularized cardiac transplant model of chronic rejection. Allograft rejection was remarkably accelerated in B6 background B7DKO and CD28KO recipients compared with B6 wild-type (WT) recipients. Allograft rejection was associated with a significantly enhanced Th1/Th2 alloreactivity and marked reduction in the ratio of regulatory T cells to CD4(+) effector/memory cells. We noted that administration of anti-B7-1 and anti-B7-2 mAb prior to transplantation also accelerated allograft rejection. Furthermore, depleting CD25(+) cells in B6 WT recipients of bm12 hearts prior to transplant also precipitated rejection at a similar rate. Neither B7/CD28 deficiency nor CD25 depletion affected graft survival in single MHC class I-mismatched (bm1 into B6) recipients. This study highlights the paradoxical functions of B7: CD28 costimulation in a MHC class II-mismatched model, in which the B7: CD28 pathway is demonstrated to be important in preventing rejection through the generation and maintenance of Tregs.
Yang J, Riella LV, Boenisch O, Popoola J, Robles S, Watanabe T, Vanguri V, Yuan X, Guleria I, Turka LA, Sayegh MH, Chandraker A.
Am J Transplant. 2009 Dec;9(12):2837-44.