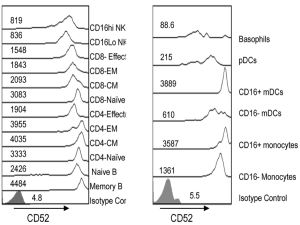
 For severe or refractory rejections, thymoglobulin/ATG is commonly employed in combination with steroids and B-cell targeted therapies when indicated. However, alemtuzumab could be more effective then thymoglobulin since it depletes not only T cells but also memory B cells, NK cells and some DCs (Figure demonstrating expression of CD52 on different immune cells; Rao et al. PLOS One 2012).
For severe or refractory rejections, thymoglobulin/ATG is commonly employed in combination with steroids and B-cell targeted therapies when indicated. However, alemtuzumab could be more effective then thymoglobulin since it depletes not only T cells but also memory B cells, NK cells and some DCs (Figure demonstrating expression of CD52 on different immune cells; Rao et al. PLOS One 2012).
Despite the absence of randomized trials, one small study reported the use of alemtuzumab for steroid-resistant cellular rejection (van den Hoogen et al. AJT 2013). They compared adult patients with steroid-resistant renal allograft rejection that were treated with either alemtuzumab (15-30 mg s.c. on 2 subsequent days; n = 11) or rabbit ATG (2.5-4.0 mg/kg bodyweight i.v. for 10-14 days; n = 20). Although not statistically significant, 27% of alemtuzumab-treated patients experienced treatment failure compared to 40% in ATG group (p = 0.70). Incidence of infection was similar, while ATG had more significant infusion-related reactions. Few other case reports of success in treating refractory/severe rejections in kidney transplants (case report 1 and 2) and lung transplants (n=22, Reams et al. AJT 2007) are referenced here.
One of the advantages of alemtuzumab is that it can be given subcutaneously with only one or two doses and it is cheaper than ATG. We had few patients with plasma-cell rich rejections or refractory cellular rejections, in which we stained for CD52 and it was diffusely positive. We decided to administer Alemtuzumab. Due to the heterogeneity of the group, it is difficult to conclude at this time how effective it was though few patients had great responses.
In sum, alemtuzumab should be considered as an alternative therapy for severe/refractory rejection. Whether staining CD52 on biopsies would help guide us in selecting alemtuzumab in place of thymoglobulin still requires further investigation.
