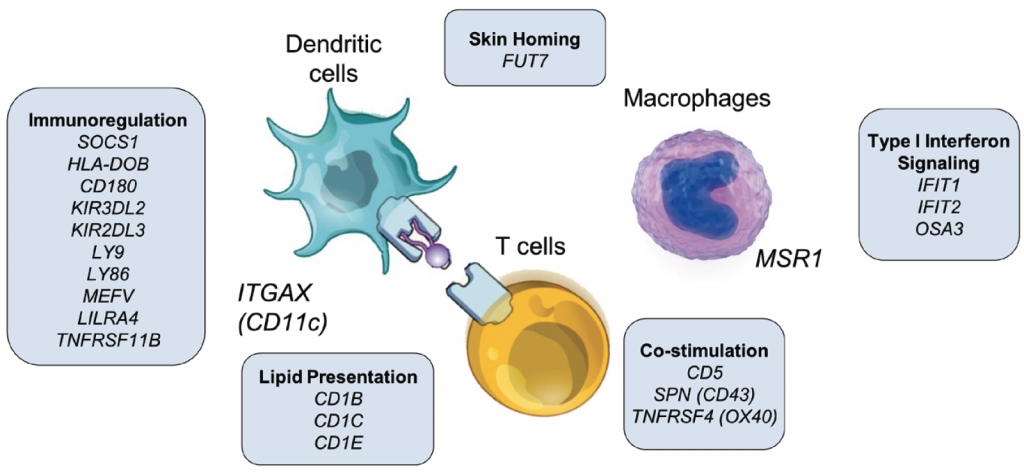
Immunoregulatory and lipid presentation pathways are upregulated in human face transplant rejection.
Background: Rejection is the primary barrier to broader implementation of vascularized composite allografts (VCA), including face and limb transplants. The immunologic pathways activated in face transplant rejection have not been fully characterized.
Methods: Utilizing skin biopsies prospectively collected over nine years from seven face transplant patients, we studied rejection by gene expression profiling, histology, immunostaining and T cell receptor sequencing.
Results: Grade 1 rejection did not differ significantly from non-rejection, suggesting that it does not represent a pathologic state and that watchful waiting is warranted. In Grade 2, there was a balanced upregulation of both pro-inflammatory T cell activation pathways and anti-inflammatory checkpoint and immunomodulatory pathways, with a net result of no tissue injury. In Grade 3, IFNγ-driven inflammation, antigen presenting cell activation and infiltration of the skin by proliferative T cells bearing markers of antigen specific activation and cytotoxic effector molecules tipped the balance towards tissue injury. Rejection of VCA and solid organ transplants had both distinct and common features. VCA rejection was uniquely associated with upregulation of immunoregulatory genes, including SOCS1, induction of lipid antigen-presenting CD1 proteins, and infiltration by T cells predicted to recognize CD1b and CD1c.
Conclusions: Our findings suggest that the distinct features of VCA rejection reflect the unique immunobiology of skin and that enhancing cutaneous immunoregulatory networks may be a useful strategy in combatting rejection.
Figure. Genes unique to face transplant rejection. A subset of genes is shown; the complete list of unique genes is shown in Supplementary Table 13