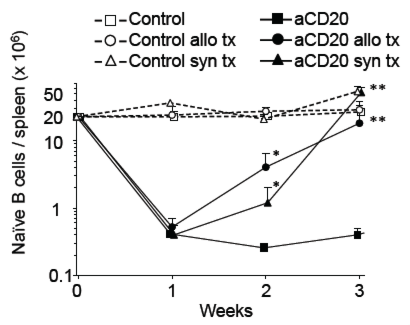
B cells play a central role in antibody-mediated rejection and certain auto-immune diseases. However, B-cell-targeted therapy such as anti-CD20 B cell-depleting antibody(aCD20) has yielded mixed results in improving outcomes. In this study, we investigated whether an accelerated B-cell reconstitution leading to aCD20 depletion-resistance could account for these discrepancies. Using a transplantation model, we found that antigen-independent inflammation, likely through TLR signals, was sufficient to mitigate B cell depletion. Secondary lymphoid organs had a quicker recovery of B cells when compared to peripheral blood. Inflammation altered the pharmacokinetics(PK) and pharmacodynamics(PD) of aCD20 therapy by shortening drug half-life and accelerating the reconstitution of the peripheral B-cell pool by bone marrow-derived B cell precursors. IVIG co-administration also shortened aCD20 drug half-life and led to accelerated B cell recovery. Repeated aCD20 dosing restored B cell depletion and delayed allograft rejection, especially B cell-dependent, antibody-independent allograft rejection. These data demonstrate the importance of further clinical studies of the PK/PD of monoclonal antibody treatment in inflammatory conditions and highlight the disconnect between B cell depletion on peripheral blood and on secondary lymphoid organs, the deleterious effect of IVIG when given with aCD20, and the relevance of re-dosing of aCD20 for effective B cell depletion in alloimmunity.
Lindsay H. Laws1*, Clare E. Parker1*, Ganesh Cherala2, Yoshinobu Koguchi3, Ari Waisman4, Mark K. Slifka5, Martin H. Oberbarnscheidt6, Jagdeep S. Obhrai1, Melissa Y. Yeung7, †, Leonardo V. Riella 7, †
American Journal of Transplantation 2016 2016 Jun 6. doi: 10.1111/ajt.13902. [Epub ahead of print]