Authors: Magee CN, Murakami N, Borges TJ, Shimizu T, Safa K, Ohori S, Cai S, Uffing A, Azzi J, Elyaman W, Charbonnier LM, Liu K, Toprak D, Visner G, Chatila TA, Siebel CW, Najafian N, Riella LV.
Abstract:
BACKGROUND:
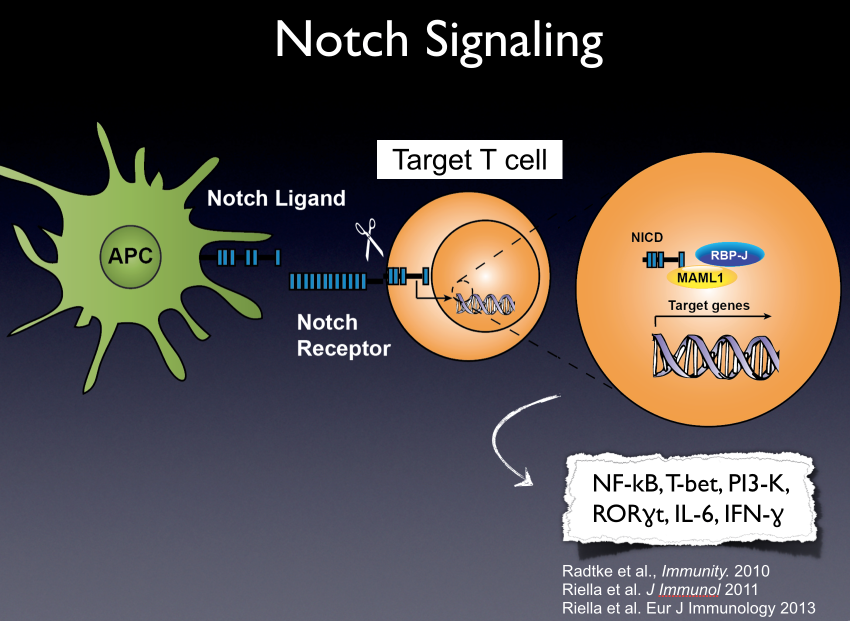
Transplantation is the treatment of choice for many patients with end-stage organ disease. Despite advances in immunosuppression, long-term outcomes remain suboptimal, hampered by drug toxicity and immune-mediated injury, the leading cause of late graft loss. The development of therapies that promote regulation while suppressing effector immunity is imperative to improve graft survival and minimize conventional immunosuppression. Notch signaling is a highly conserved pathway pivotal to T cell differentiation and function, rendering it a target of interest in efforts to manipulate T cell-mediated immunity.
METHODS:
We investigated the pattern of Notch-1 expression in effector and regulatory T cells (Tregs) in both murine and human recipients of a solid organ transplant. Using a selective human anti-Notch-1 antibody (aNotch-1), we examined the effect of Notch-1 receptor inhibition in full MHC-mismatch murine cardiac and lung transplant models, and in a humanized skin transplant model. Based on our findings, we further employed a genetic approach to investigate the effect of selective Notch-1 inhibition in Tregs.
RESULTS:
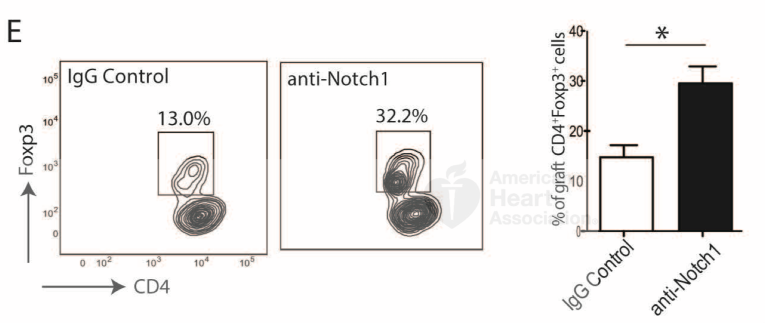
We observed an increased proportion of Tregs expressing surface and intracellular (activated) Notch-1 compared to conventional T cells (Tconv), both in transplanted mice and in the peripheral blood of transplanted patients. In the murine cardiac transplant model, peri-transplant administration of aNotch-1 (days 0, 2, 4, 6, 8, 10) significantly prolonged allograft survival compared to IgG-treated controls. Similarly, aNotch-1 treatment improved both histological and functional outcomes in the murine lung transplant model. The use of aNotch-1 resulted in a reduced proportion of both splenic and intra-graft Tconv, while increasing the proportion of Tregs. Furthermore, Tregs isolated from aNotch-1 treated mice showed enhanced suppressive function on a per-cell basis, confirmed with selective Notch-1 deletion in Tregs (Foxp3EGFPCreNotch1fl/fl). Notch-1 blockade inhibited the mTOR pathway and increased the phosphorylation of STAT5 in murine Tregs. Notch-1low Tregs isolated from human peripheral blood exhibited more potent suppressive capacity than Notch-1high Tregs. Lastly, the combination of aNotch-1 with costimulation blockade induced long-term tolerance in a cardiac transplant model, and this tolerance was dependent on CTLA-4 signaling.
CONCLUSIONS:
Our data reveal a promising, clinically relevant approach for immune modulation in transplantation by selectively targeting Notch-1.