Current anti-rejection medications can cause serious side effects. Our research focuses on finding safer ways to protect transplanted organs by teaching the immune system to accept them naturally.
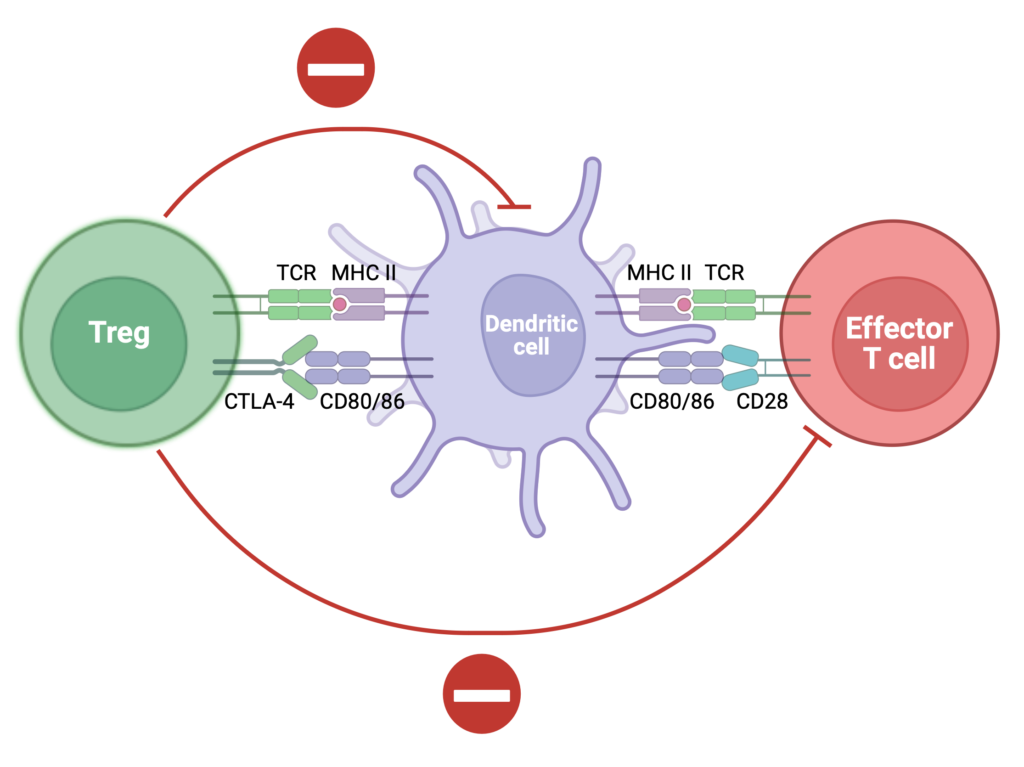
We study how special immune cells—like regulatory T cells—can calm the immune response and prevent rejection. Our team helped develop a modified IL-2 protein that boosts these protective cells and improves transplant survival (JCI 2024).
We also discovered a natural “brake” in the immune system—called Siglec-E—that helps prevent inflammation and rejection (Science Translational Medicine 2025).
Our goal is to develop smarter, targeted therapies that reduce the need for lifelong medications and help patients live longer, healthier lives after transplant.
See also some of our groundbreaking research in the following publications at Circulation, Nature Comm, JCI, JCI Insight, American Journal Transp, and Transplantation.
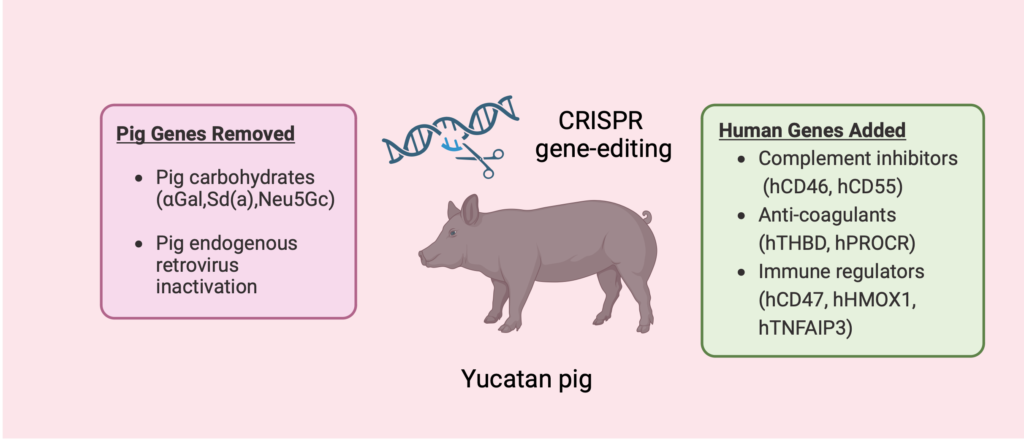
We are addressing the critical shortage of donor kidneys through two transformative strategies: growing bioengineered kidneys from human stem cells (iScience 2024) and advancing gene-edited pigs for human transplantation.
Our group has led landmark first-in-human studies reporting clinical outcomes of living human kidney xenotransplantation (New England Journal of Medicine), defining kidney xenograft physiology in humans (Nature Communications), and characterizing the human immune response to pig kidneys (Nature Medicine).
Together, these complementary clinical and mechanistic insights are shaping next-generation immunosuppressive strategies, guiding donor genetic engineering, and accelerating xenotransplantation toward a scalable alternative to dialysis.
Additional related publications: Harvard, JASN, Xenotransp, Transp, Ann Surg, Xenotransp, Am J Transp 2025).
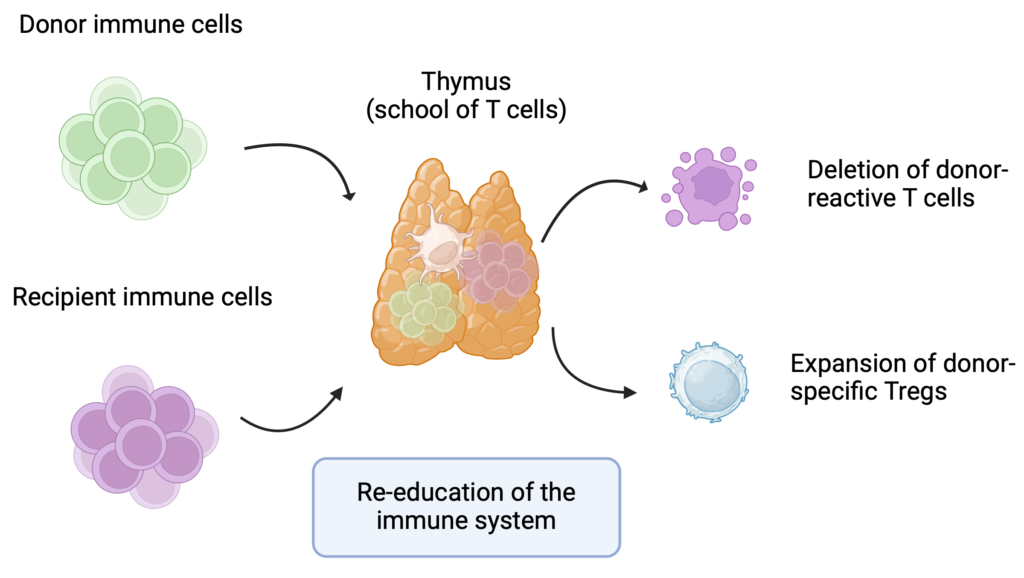
Preventing organ rejection without the need for lifelong immunosuppressive drugs is often called the “Holy Grail” of transplantation. At Massachusetts General Hospital, we established the Legorreta Center for Clinical Transplant Tolerance —the first center of its kind in the world.
Through innovative protocols combining kidney and bone marrow transplantation, we are offering patients a new path to achieving long-term transplant acceptance with minimal or no immunosuppression.
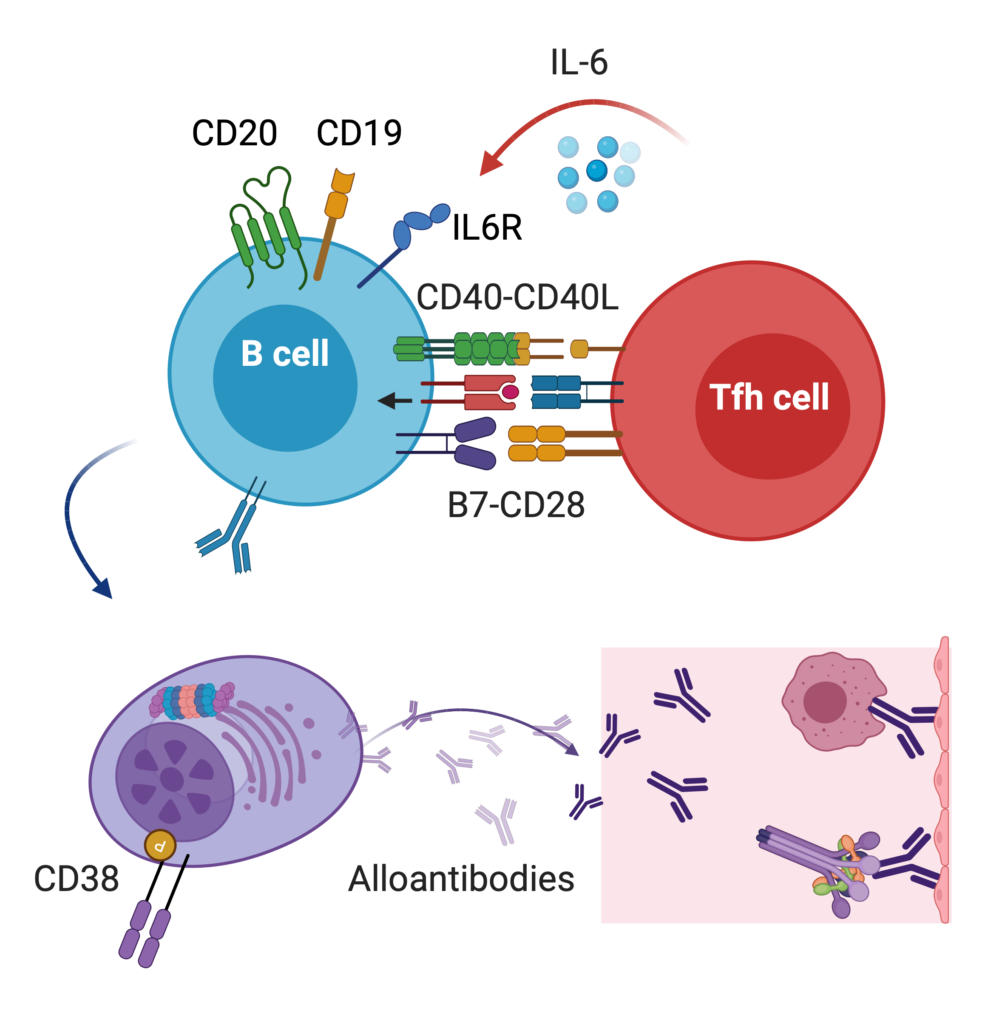
Antibody-mediated rejection is the leading cause of kidney loss after transplantation. We are studying how antibodies cause injury post-transplant (antibody glycosylation) and how to use the regulatory mechanisms of the immune system to shut off antibody production. We have unique strategies and protocols to deal with highly sensitized patients (Frontiers, Transp). Read our reviews Curr Opin Organ Transplant and AJT
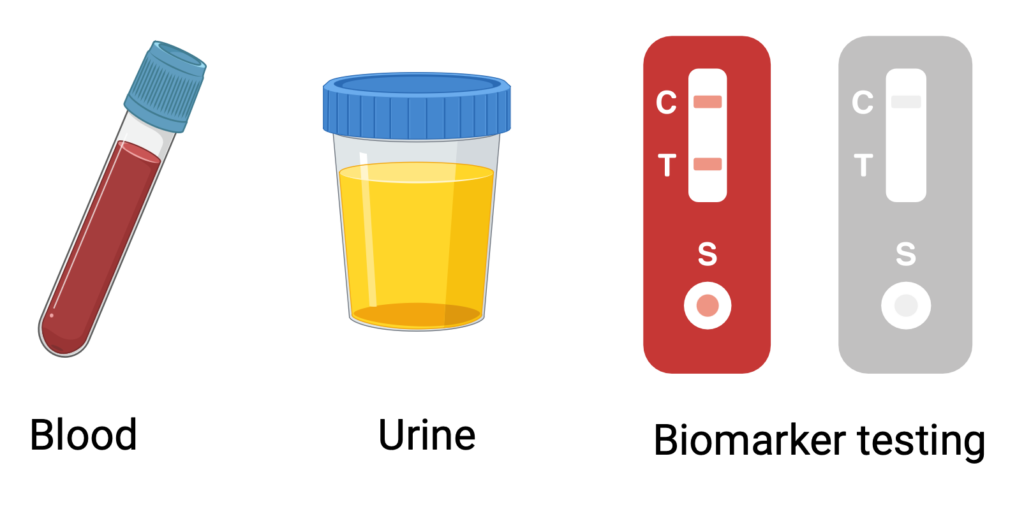
Currently, detecting issues with transplanted organs often requires costly and invasive tests, such as biopsies. At our center, we use a unique biobank of human transplant samples to study each patient’s genetics, clinical profile, and blood and urine biomarkers, aiming to tailor and personalize transplant care (JCI, JCI Insight). Among our innovations, we have developed a novel, affordable, and highly sensitive urine test that uses CRISPR/Cas13 technology to detect early signs of rejection (Nat Biomed Eng 2020). We’ve also adapted the CRISPR/Cas platform to identify genetic mutations, further advancing precision in transplant monitoring (EMBO 2024). Additionally, we’re exploring how wearable devices can help detect transplant-related complications earlier, enhancing proactive patient care.
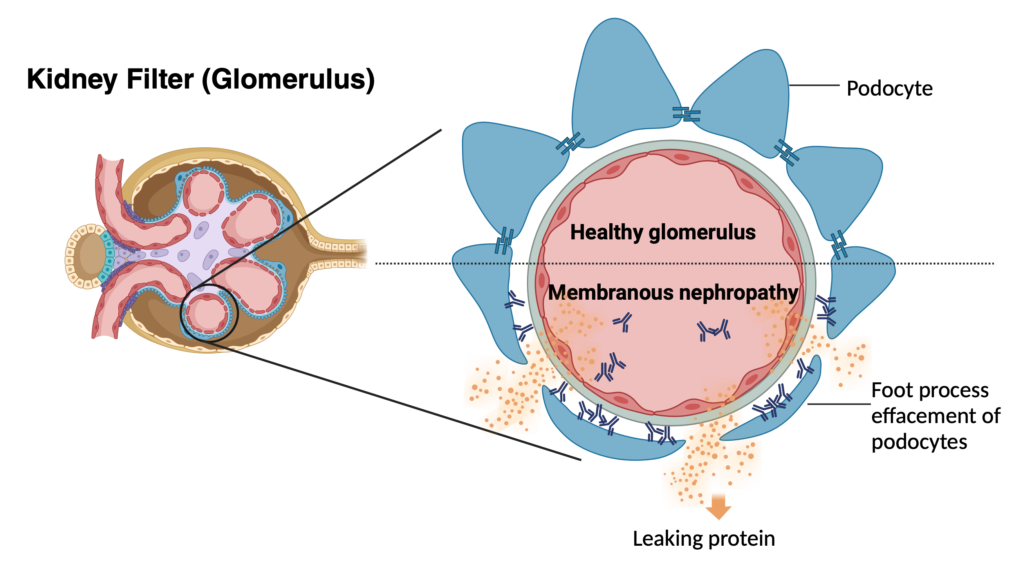
Recurrence of kidney disease is a leading cause of kidney transplant loss. To tackle this problem, we have established the largest collaborative international effort to study glomerular disease recurrence, the TANGO study. The goal of TANGO is to better understand the underlying causes of recurrence (genetic, environmental, immune dysregulation). By employing detailed genetic testing, clinical information and immune characterization, we’re developing tailored treatments to prevent disease recurrence and extend the longevity of transplants. Check out our work on FSGS, IgA and membranous nephropathy recurrence; also our review on CJASN.
Most recently, in KI Reports, we showed that patients with primary glomerular diseases are frequently excluded from kidney transplant clinical trials and proposed a risk-stratified framework to promote more equitable and evidence-based trial inclusion.
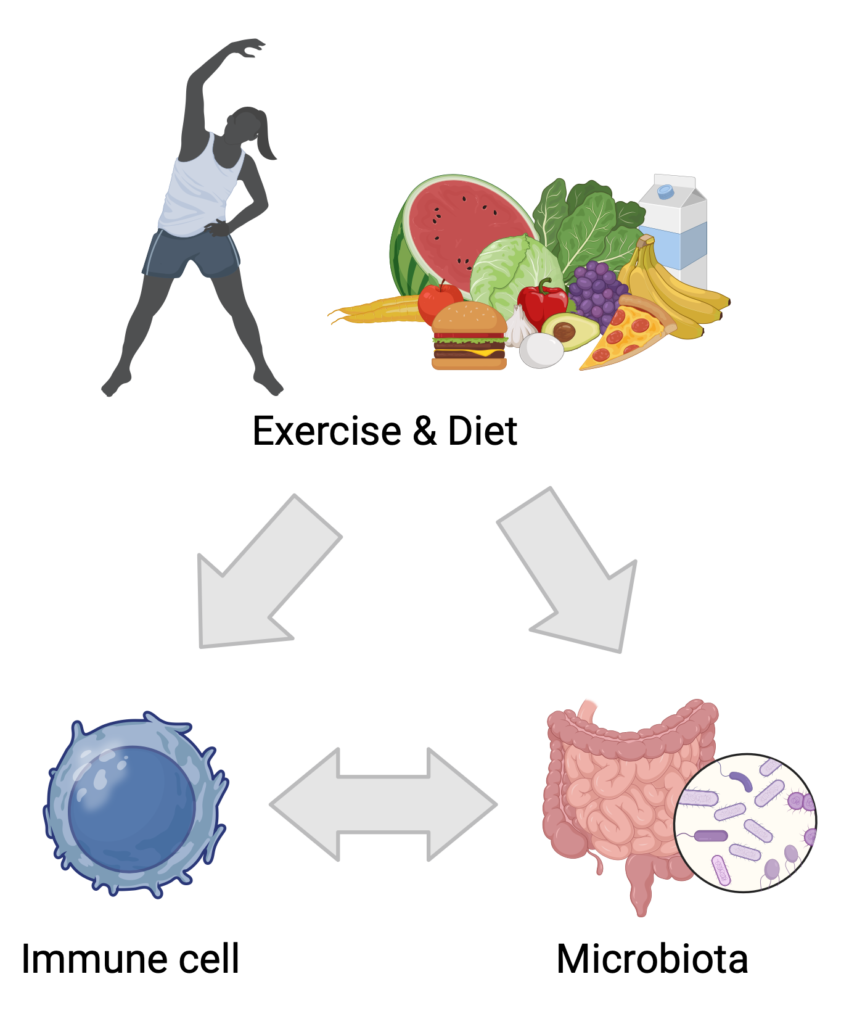
Our research explores how dietary patterns and physical activity influence both the gut microbiota and immune responses in transplant recipients (JCI review). By investigating these connections, we aim to identify actionable strategies that support immune regulation, reduce the risk of rejection, and enhance long-term health. For example, our study published in JASN demonstrated that high salt intake impairs regulatory T cell function and promotes pro-inflammatory responses, highlighting how excess dietary salt can contribute to immune dysregulation and potentially worsen transplant outcomes. The GENIE study (Front Immunol) evaluated the anti-inflammatory properties of dietary modifications in patients with kidney disease, offering insight into how nutrition can modulate immune pathways even prior to transplantation. Additional work has examined the benefits of structured exercise programs in kidney disease (Front Physiol) and developed evidence-based dietary recommendations tailored to kidney transplant recipients (Kidney Int Rep). Together, these findings support a holistic and personalized approach to post-transplant care.
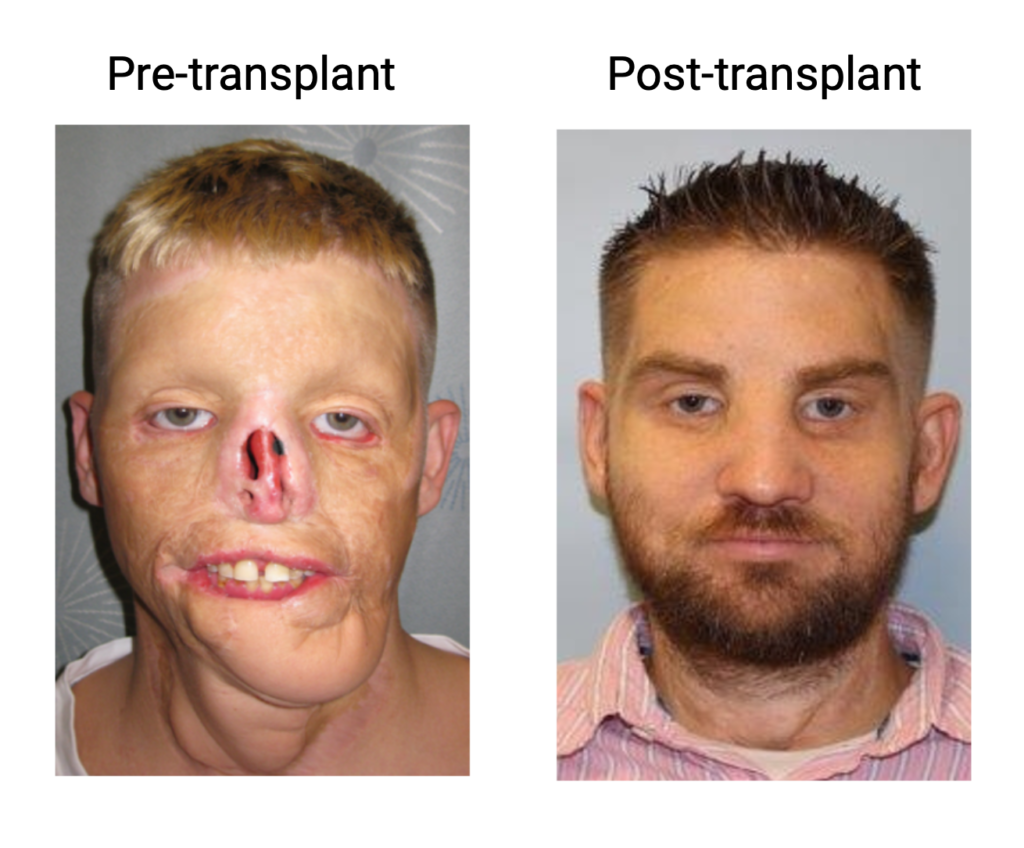
For patients facing severe body injuries, traditional reconstruction options can be insufficient. Our team is at the forefront of vascular composite tissue transplantation (VCA), leveraging animal models, advanced multiomics platforms, and a unique biobank from human recipients of face and limb transplants (eg. Am J Transp 2016, Nat Scientific Report 2018, NEJM 2019, J Clin Invest. 2021, Cell Report 2022, AJT 2023, AJT 2025). Our goal is to deepen our understanding of the underlying biology, identify novel biomarkers, and improve outcomes for those undergoing limb and facial transplantation.
In the face of COVID-19, solid organ transplant recipients emerged as a particularly vulnerable group, experiencing heightened risk of severe illness and mortality. Our lab led longitudinal and mechanistic studies defining immune responses to SARS-CoV-2 vaccination in transplant recipients, including antibody and cellular responses after repeated mRNA vaccine doses (AJT 2020; Kidney Int 2022; Transplantation 2023) and the durability of protection against emerging Omicron variants.
We have also investigated preventive strategies, demonstrating that tixagevimab–cilgavimab pre-exposure prophylaxis reduced breakthrough infections in transplant recipients, with variant-specific limitations (AJT 2022; Transplantation 2023). More recently, we characterized post-acute sequelae of COVID-19 during the Omicron period, identifying persistent symptoms and clinical impact unique to transplant recipients (Transplant Direct 2024). Together, this work informs precision vaccination strategies, prophylactic interventions, and long-term management of COVID-19 in immunocompromised patients.