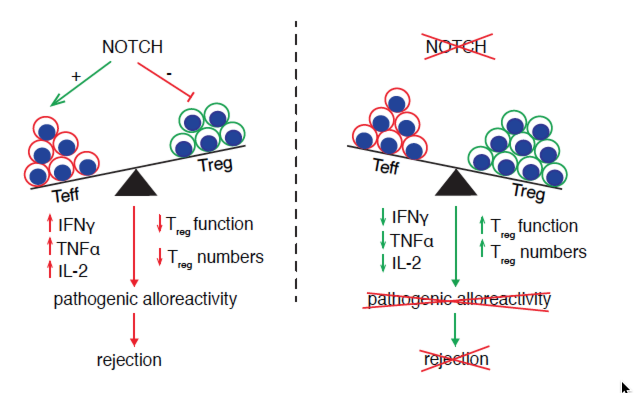
Immune rejection is mediated by a complex interplay of cellular and humoral mechanisms. Current therapeutic strategies, which rely on global immunosuppression, can result in serious complications and are not completely effective. Notch signaling is a cell-to-cell communication pathway that plays an important role during T cell development and in the regulation of peripheral immune responses. Initial work, performed mainly through gain-of-function approaches, paradoxically identified Notch as an inducer of tolerance. However, recent studies using loss-of-function approaches in mouse models of transplant rejection and graft-versus-host-disease have clarified an important role for Notch as a central mediator of T cell alloreactivity. Short-term inhibition of individual Notch ligands in the peri-transplant period had long-lasting protective effects. In a vascularized heart allograft model, blockade of Delta-like Notch ligands dampened both cellular and humoral rejection. Here, we summarize current knowledge on the role of Notch signaling during allograft rejection, and provide an overarching mechanism through which Notch acts to promote T cell pathogenicity and allograft damage. We propose that targeting elements of the Notch pathway could provide a new therapeutic approach to prevent allograft rejection. This article is protected by copyright. All rights reserved.