KIDNEY360 1: 130–140, 2020.
Shruti Gupta , Frank B. Cortazar, Leonardo V. Riella, and David E. Leaf
Full PDF version on this link
Abstract
Immune checkpoint inhibitors (ICPIs) have transformed the landscape of oncology, but are associated with a variety of autoimmune adverse events, including AKI. ICPI-associated AKI (ICPI-AKI) is emerging as an increasingly frequent cause of AKI in patients with cancer, and poses unique diagnostic and management challenges to clinicians who care for these patients. In this review, we describe the incidence and risk factors for ICPI-AKI, including proton pump inhibitor use, CKD, and combination immunotherapy. We discuss the limitations of the various definitions used for ICPI-AKI in prior studies, and propose a novel classification system (definite, probable, and possible ICPI-AKI) that recognizes the diagnostic uncertainty inherent in many cases. We discuss the key clinicopathologic features and treatment strategies for ICPI-AKI, including the role of kidney biopsy versus empirical treatment with steroids. We also explore the under-studied area of ICPI use in the setting of solid organ transplantation, where nephrologists and oncologists must balance the risk of rejection versus treating the underlying malignancy. Finally, we summarize existing data on the role of ICPI rechallenge after an episode of ICPI-AKI.
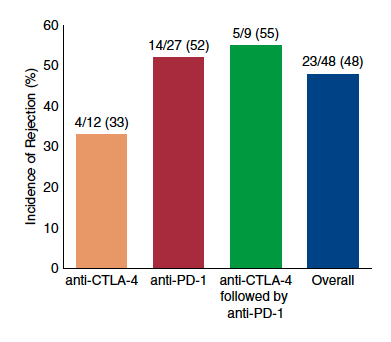
Figure. Incidence of solid organ transplant rejection may be higher after anti–PD-1 therapy. Data are derived from published case series and case reports of rejection after immune checkpoint inhib- itors. CTLA-4, cytotoxic T lymphocyte–associated protein 4; PD-1, programmed cell death protein 1; PD-L1, PD-ligand 1.