Transplantation. 2023 Jun 5. doi: 10.1097/TP.0000000000004697. Online ahead of print.
We extended our prior retrospective multicenter cohort study1 to include all SOTRs at our institutions who received their first tixagevimab–cilgavimab dose between December 28, 2021, and June 30, 2022. A control group of age-matched SOTRs who did not receive tixagevimab– cilgavimab was used for comparison. The primary outcome was the development of any breakthrough SARS-CoV-2 infection during the period of BA.5 predominance, defined as a newly positive SARS-CoV-2 antigen or polymerase chain reaction test between July 2, 2022, and November 4, 2022, performed for symptoms or other indications.
Six hundred thirty-three SOTRs received tixagevimab– cilgavimab at our institutions between December 28, 2021, and June 30, 2022. Baseline characteristics were similar between the tixagevimab–cilgavimab and control groups, except for a higher proportion of SOTRs with a history of prior SARS-CoV-2 infection, a higher rate of unvaccinated individuals, a lower proportion of lung transplant recipients, and a longer time since transplantation in the control group (Table 1).
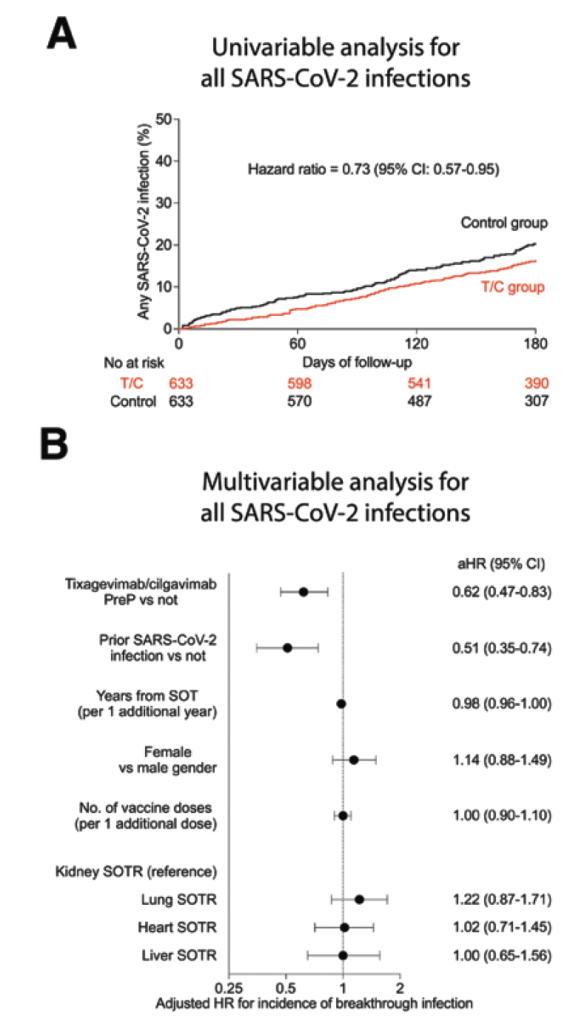
Univariable and multivariable analyses showed that tixagevimab–cilgavimab PreP was associated with a lower risk of developing any breakthrough SARS-CoV-2 infection (unadjusted hazard ratio [HR] = 0.73; 95% confidence interval [CI], 0.57-0.95 and adjusted HR [aHR] = 0.62; 95% CI, 0.47-0.83, respectively; Figure 1A and B). There was no significant association between tixagevimab–cilgavimab PreP and breakthrough SARS-CoV-2 infection resulting in hospitalization or death in univariable (unadjusted HR = 0.56; 95% CI, 0.28-1.11; Figure 1C) or multivariable analysis (aHR = 0.57; 95% CI, 0.28- 1.15; Figure 1D). Multivariable analysis also showed that additional vaccine doses (aHR = 0.76 for each additional vaccine dose; 95% CI, 0.61-0.95) and prior SARS-CoV-2 infection (aHR = 0.28; 95% CI, 0.09-0.92) were associated with a significantly lower risk of hospitalization or death from SARS-CoV-2 infection. During the BA.5 period, there was no significant association between tixagevimab–cilgavimab PreP and the incidence of breakthrough SARS-CoV-2 infection.
FIGURE 1. T/C PreP is associated with a lower risk of SARS-CoV-2 infection in SOTRs, except during the BA.5 period. A, Univariable and (B) multivariable analyses for the association of T/C PreP with the risk of any breakthrough SARS-CoV-2 infection in SOTRs (n=1266).